Blog Post
Trends in Clinical Trial Participation of Approved Drugs by Sex and Race: 2015 to 2019
February 27, 2020
 Yael Symes, PhD, Integrated Product Development Associate, has over 10 years of experience in writing and editing scientific documents, including 9 publications in peer-reviewed journals. She has participated in the authoring and preparation of clinical protocols, modules of NDAs and INDs, clinical study reports, and other regulatory documents in a variety of therapeutic areas. Dr. Symes received her PhD in Health Behavior at the University of North Carolina at Chapel Hill Gillings School of Global Public Health.
Yael Symes, PhD, Integrated Product Development Associate, has over 10 years of experience in writing and editing scientific documents, including 9 publications in peer-reviewed journals. She has participated in the authoring and preparation of clinical protocols, modules of NDAs and INDs, clinical study reports, and other regulatory documents in a variety of therapeutic areas. Dr. Symes received her PhD in Health Behavior at the University of North Carolina at Chapel Hill Gillings School of Global Public Health.
In 2012, Congress directed the Food and Drug Administration (FDA) to investigate how well demographic subgroups are included in clinical trials in applications for medical products (FDASIA 907). The FDA responded by creating the Drug Trials Snapshots annual summary report to make demographic subgroup data more available and transparent. Drug Trials Snapshots summary reports include demographic data from pivotal clinical trials used to approve novel drugs (i.e., new molecular entities and original biologic products) each year by sex and race/ethnicity subgroups.
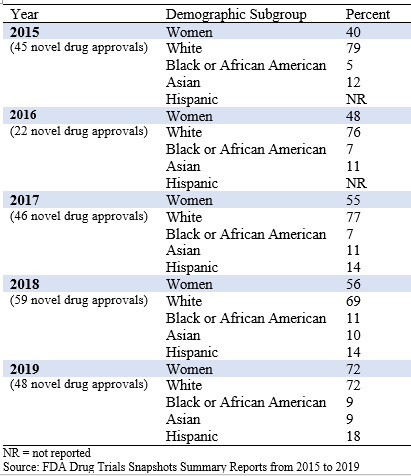
Table 1 presents demographics by sex and race/ethnicity from 2015 to 2019.
Table 1: Demographic Summaries of Novel Drug Approvals from 2015 to 2019
Trends by Sex
In 2015, 40% of clinical trial participants for the novel drugs approved that year were women compared with 72% in 2019. This increase may be due to drug developers’ efforts to recruit more women in clinical trials, but it also reflects the drugs that were approved specifically for women, including Vyleesi for treatment of hypoactive sexual desire disorder and Zulresso for treatment of postpartum depression. In 2019, 6 novel drugs were approved specifically for women compared with 2 drugs approved for men or boys.
Trends by Race/Ethnicity
In 2015, 79% of clinical trial participants for novel drugs approved that year were White, 5% were Black or African American, and 12% were Asian. FDA did not report Hispanic subgroups until 2017. In 2019, 72% of clinical trial participants for novel drugs approved that year were White, 9% were Black or African American, 9% were Asian, and 18% were Hispanic, which generally mirrored the 2019 demographics of the U.S. population (77% White, 13% Black or African American, 6% Asian, and 18% Hispanic; U.S. Census Bureau, 2019).
However, racial and ethnic minority participation varied widely by therapeutic area. In approvals for novel oncology drugs in 2019, 4% of study participants were Black or African American, 5% were Hispanic, and 18% were Asian. African Americans are more likely to be diagnosed with certain cancers and are more likely to die from some cancers compared with White Americans (American Cancer Society, 2019). It is important for clinical trial populations to reflect the racial and ethnic demographics of the intended treatment population.
Conclusions
Women and racial and ethnic minority populations have historically been excluded from clinical trials (Goldstein & Walensky, 2019; Liu & Mager, 2016). In 1977, the FDA discouraged including women in clinical research except for drugs tested to treat life-threatening conditions because they were considered a vulnerable population. The FDA reversed this stance in 1993 (FDA, 1993) and in 2019, the FDA released a draft guidance on enhancing the diversity of clinical trial populations (FDA, 2019). In the 2019 draft guidance, the FDA urged product developers to adopt practices that will allow clinical trial populations to reflect the diversity of patients who will use the product if it is approved.
Diversity in clinical research is important for ethical and scientific reasons. When women and non-White populations are not represented in clinical research, this limits our understanding of the safety and efficacy of drugs to treat important health issues in these populations. It is encouraging that women have become better represented in clinical research over time; however, there is still room for improvement to increase racial and ethnic diversity in clinical research, particularly in oncology. There are tangible steps that product developers can take to improve diversity in clinical research, including actions to reduce subject burden and foster trust (Symes & Modell, 2019).
References:
American Cancer Society. Cancer Facts & Figures 2019.
https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf
Atlanta: American Cancer Society; 2019.Goldstein RH & Walensky, RP. (2019). Where Were the Women? Gender Parity in Clinical Trials. New England Journal of Medicine, 381(26), 2491-2493.
Liu, KA & Mager, NAD. (2016). Women’s involvement in clinical trials: historical perspective and future implications. Pharmacy Practice. 14(1), 708.
Symes YS & Modell, J. (2020). What Drug Development Sponsors, Contract Research Organizations, and Investigators Can Do to Increase Diversity in Clinical Trials. Journal of Clinical Pharmacology. 60(3), 281-283.
U.S. Census Bureau. Quick Facts United States: Population Estimates, July 1 2018. https://www.census.gov/quickfacts/fact/table/US/PST045218
U.S. Food and Drug Administration (1993). Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs, 58:39406–39416. https://www.fda.gov/media/75648/download
U.S. Food & Drug Administration. 2015-2016 Drug Trials Snapshots.
https://www.fda.gov/media/103160/download
U.S. Food & Drug Administration. 2017 Drug Trials Snapshots Summary Report.
https://www.fda.gov/media/112373/download
U.S. Food & Drug Administration. 2018 Drug Trials Snapshots Summary Report.
https://www.fda.gov/media/120253/download
U.S. Food and Drug Administration (2019). Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrollment Practices, and Trial Designs Draft Guidance for Industry.
https://www.fda.gov/media/127712/download
U.S. Food & Drug Administration. 2019 Drug Trials Snapshots Summary Report.
https://www.fda.gov/media/135337/download
